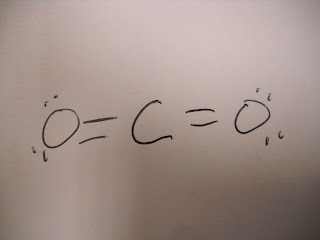+of+Structure-C2H4.JPG)
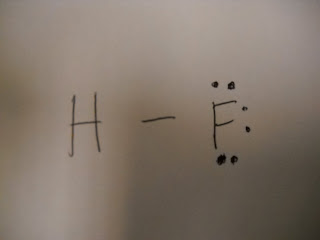



molecule model lab
Title: Polarity and Molecular Shape Lab
Statement of the Problem:
How shape affects polarity.
How elements combine to form molecules.
Hypothesis:
We hypothesize that based on the shape of a molecule we can determine whether or not the molecule is polar.
Materials:
a. Ball and stick model sets, pencils, paper, molecular shape chart, and a camera.V. Introduction:
Because of the principle of outer electron shell repulsion, the electronegativity difference determines which type of bond any set of elements may engage in. For example, if the difference is more than 2.5 the bond is ionic if between .5 and 2.5 polar covalent, and if less than .5 nonpolar covalent. This repulsion also means that atoms in a bond will push one another as far away as is possible within the given bond.
VI. Procedure:
Because of the principle of outer electron shell repulsion, the electronegativity difference determines which type of bond any set of elements may engage in. For example, if the difference is more than 2.5 the bond is ionic if between .5 and 2.5 polar covalent, and if less than .5 nonpolar covalent. This repulsion also means that atoms in a bond will push one another as far away as is possible within the given bond.
VI. Procedure:
We drew a Lewis structure based on the chemical formula.
Using the shape chart we determined the actual molecular shape.
We then made a model based on the actual molecular shape and the Lewis structure that we made.
Then we proceeded to determine if the molecule could be polar based on the shape of the molecule.
We then drew the model and took several pictures of the models.
VI. Results:
See top of page.
Conclusion:
This lab is great for anyone who is a spacial learner. It helps them visualize what really happens with bonds.